MPOX Vaccines
- ACIP reviewed evidence to recommendations framework and included updates to US mpox epidemiology, vaccine safety and vaccine effectiveness.
- ACIP recommends vaccination with the 2-dose Smallpox and Monkeypox (Live, Non-replicating) JYNNEOS vaccine series for persons aged 18 years and older at risk for mpox. Interim recommendation to be revisited in 2-3 years.
- Persons at risk for mpox
- Gay, bisexual, and other men who have sex with men, transgender or nonbinary people who in the past 6 months have had one of the following:
- A new diagnosis of ≥1 sexually transmitted disease
- More than one sex partner
- Sex at a commercial sex venue
- Sex in association with a large public event in a geographic area where mpox transmission is occurring
- Sexual partners of persons with the risks described in above
- Persons who anticipate experiencing any of the above
- VOTE Result: PASSED (Unanimous); Vaccines for Children VOTE Result: PASSED (Unanimous)
RSV Vaccines
Some of the items addressed by ACIP included:
- Epidemiology of hospitalization in 50-59 aged adults
- RSV adult work group considerations
Influenza Vaccines
Three individual studies with data on the use of influenza vaccines in pregnant women were presented.
- Safety of quadrivalent recombinant influenza vaccine (RIV4) vs standard dose (SD-IIV4) in pregnant women and their infants1
- No statistically significant differences in pregnancy, birth, and neonatal/infant outcomes between RIV4 and SD-IIV4
- Pregnancy outcomes with cell culture influenza vaccine (ccIIV4)2
- No evidence of a safety concern with use of ccIIV4 in pregnant women
- Effectiveness of maternal influenza vaccination during pregnancy against influenza-associated hospitalizations & ED visits in infants <6 months of age3
- Maternal vaccination was associated with reduced odds of influenza hospitalizations & ED visits in infants <6 months of age
- Vaccine effectiveness was greatest amongst infants <3 months of age, those born to mothers vaccinated during their third trimester of pregnancy, and against influenza-associated hospitalizations
Two individual studies with data on concomitant administration of influenza vaccines were presented.
- A study on safety of simultaneous vs sequential administration of mRNA COVID-19 and quadrivalent influenza vaccine (IIV4)4 demonstrated that simultaneous administration was well-tolerated when compared to sequential administration
- A study on safety of simultaneous vaccination with zoster vaccine recombinant (RZV) and quadrivalent adjuvanted vaccine (aIIV4)5 showed no statistically significant differences in frequency of severe local or systemic reactions in participants receiving one dose of RZV and either aIIV4 or a high-dose quadrivalent influenza vaccine (HD-IIV4)
The WHO’s Global Influenza Surveillance and Response System identified zero confirmed circulating B/Yamagata lineage viruses.6
- The WHO influenza vaccine composition advisory committee states that inclusion of a B/Yamagata strain in vaccines is no longer warranted and should be excluded as soon as practically possible
Influenza Vaccines References
- ACIP Presentation. Klein N. 25 Oct 2023. Safety of quadrivalent recombinant influenza vaccine in pregnant women and their infants. (Link)
- ACIP Presentation. Sylvester G. 26 Oct 2023. Pregnancy outcomes with ccIIV4 (Flucelvax): results of a post-marketing study. (Link)
- ACIP Presentation. Olson S. 26 Oct 2023. Effectiveness of maternal influenza vaccination during pregnancy against influenza-associated hospitalizations & emergency department visits in infants <6 Months of Age. (Link)
- ACIP Presentation. Walter E. 25 Oct 2023. Safety of simultaneous vs sequential administration of mRNA COVID-19 and quadrivalent inactivated influenza (IIV4) vaccines: A randomized placebo-controlled trial. (Link)
- ACIP Presentation. Schmader K. 25 Oct 2023. Safety of simultaneous vaccination with zoster vaccine recombinant (RZV) and quadrivalent adjuvanted inactivated influenza vaccine (aIIV4). (Link)
- ACIP Presentation. Kondor R. 26 Oct 2023. Update on Influenza B/Yamagata Surveillance (Link)
Immunization Schedules
- The Combined Immunization Schedule Work Group provided overview of the updates to the immunization schedules for 2024.
- Traditional publication timeline in February of each year has led to delays in insurance reimbursement, administration authority (e.g., pharmacists) and health care provider knowledge and practices related to vaccine recommendations.
- A new “Addendum” page at the end of the schedules will include ACIP recommendations that occur after the 2024 schedule is published. With this addition, all ACIP recommendations will formally be part of the CDC Immunization Schedules.
- Under new shorter publication timeline for 2024 schedules, schedules will be published online in November 2023. MMWR and Annals of Internal Medicine report will be published in December-January 2024.
- VOTE Results: PASSED
Chikungunya Vaccines
ACIP reviewed the Evidence-to-Recommendation framework for a live-attenuated chikungunya virus vaccine, and the Chikungunya Vaccines Work Group proposed draft policy recommendations for the vaccine’s use in for laboratory workers and adult travelers. No vote was taken.
Draft recommendations ACIP is considering:
- Chikungunya vaccination is recommended for laboratory workers with potential for exposure to chikungunya virus.
- Chikungunya vaccine is recommended for persons aged ≥18 years traveling to a country or territory where there is a chikungunya outbreak.
- In addition, chikungunya vaccine may be considered for the following persons traveling to a country or territory without an outbreak but with evidence of chikungunya virus transmission among humans within the last 5 years
- Older persons (e.g., >65 years), particularly those with underlying medical conditions, who are likely to have at least moderate exposure to mosquitoes.
- Persons staying for a cumulative period of 6 months or more during a 2-year period.
Dengue Vaccines
The Chair of the ACIP Dengue Vaccine Work Group (WG) announced that, due to Takeda’s voluntary withdrawal of its BLA for dengue vaccine candidate (TAK-003), the WG will be paused until a BLA for TAK-003 is re-submitted to FDA or a BLA for another dengue vaccine candidate is submitted for approval.
COVID-19 Vaccines
- All anticipated updated (2023-2024 formula) vaccines are now authorized or approved.
- Updates to the Interim Clinical Considerations or Use of COVID-19 Vaccines in the United States include new guidance for children who transition from one age group to the next during the initial COVID-19 vaccination series and a clarification of circumstances in which administration of COVID-19 vaccine doses from different manufacturers may be considered.
- Policy discussions anticipated for February 2024 ACIP meeting include consideration of additional COVID-19 vaccine doses in older adults reaching six months since their last dose.
- Available data do not provide clear and consistent evidence of a safety problem for ischemic stroke with bivalent mRNA COVID-19 vaccines when given alone or concomitantly with influenza vaccines or when influenza vaccine is given alone.
Pneumococcal Vaccines
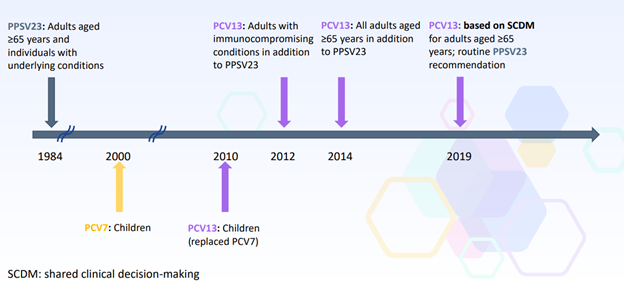
The session included a review of ACIP pneumococcal vaccinerecommendations through 2019…
|
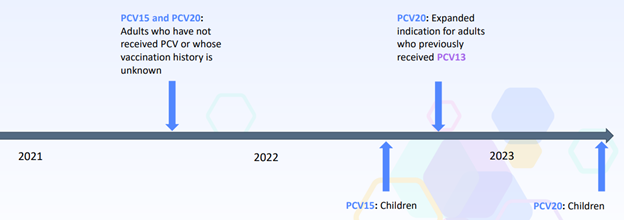
…and outlined the more recent recommendations made between 2021 to 2023:
|
A Summary of Pneumococcal Disease Burden among US Adults was presented:
- Prior to the COVID-19 pandemic it was estimated that every year pneumococcal disease caused :
-
≥100,000 non-invasive pneumococcal pneumonia hospitalizations
-
≥30,000 invasive pneumococcal disease (IPD) cases (e.g., bacteremic pneumonia, pneumococcal bacteremia, meningitis)
- Preliminary data show increase in IPD cases in late 2022
- Increase in other invasive bacterial infections reported in children
- Invasive pneumococcal disease incidence among adults aged ≥65 years reached a historically low level early in the COVID-19 pandemic
- Approximately 40% of IPD cases in adults aged ≥65 years were caused by serotypes not contained in currently recommended vaccines
Adult Pneumococcal Vaccines in Advanced Stages of Development:
-
24-valent pneumococcal vaccines
- Pn-MAPS24V, GSK
- Completed phase 1/2 study in adults
- VAX-24, Vaxcyte
- Completed phase 1/2 studies in adults, undergoing phase 2 studies in infants
-
21-valent pneumococcal conjugate vaccine
- V116, Merck
- Completed phase 1/2 study in adults, phase 3 in adults ongoing
-
Note: this vaccine does not contain several serotypes found in currently licensed vaccines
Work Group Next Steps:
- Review evidence on use of 21-valent pneumococcal conjugate vaccine (V116) for adults
- Biologics License Application for V116 to the FDA is anticipated in Q4 of 2023, with an anticipated FDA approval in the first half of 2024
- Data to be reviewed include:
- Evidence to support EtR domains including:
- Epidemiology of pneumococcal disease among U.S. adults
- Assessment of pneumococcal disease caused by serotypes not included in V116 (but included in existing vaccines)
- Phase 2/3 clinical trial data on V116 among adults
- Feasibility and resource use involved in V116 use among adults
- Health equity considerations
- Aim to have an ACIP vote on policy options shortly after FDA approval of V116
Pneumococcal Vaccines References
- ACIP Agenda. CDC. 25 October 2023. Meeting of the advisory committee on immunization practices (ACIP). (Link)
- ACIP Presentation. (Link)
Meningococcal Vaccines
Meningococcal Session Overview1,2,3,4
- ACIP voted 10-4 in favor of recommending that Pfizer’s MenABCWY vaccine may be used when both MenACWY and MenB are indicated at the same visit
- Healthy individuals aged 16-23 years (routine schedule) when shared clinical decision-making favors administration of MenB vaccination
- Individuals aged 10 years and older at increased risk of meningococcal disease (e.g., due to persistent complement deficiencies, complement inhibitor use, or functional or anatomic asplenia) due for both vaccines
- ACIP also presented its plan to re-evaluate the current adolescent meningococcal vaccination schedule over the coming year beginning in February 2024, with a vote anticipated in February 2025. The key questions that will be considered include:
- Whether meningococcal vaccination should begin at an older age than currently recommended
- Whether the 11-12 year recommendation should change to one of shared clinical decision making
- Whether the MenB shared clinical decision making recommendation for some or all adolescents should be revisited
- We are interested in your thoughts on meningococcal vaccination. Please consider completing a short 5-minute survey available here:
SURVEY LINK
Meningococcal Vaccines References
- ACIP Agenda. CDC. 25 October 2023. Meeting of the advisory committee on immunization practices (ACIP). (Link)
- ACIP Presentation. Poehling K. 25 October 2023. ACIP Meningococcal Vaccines Work Group Introduction. (Link)
- ACIP Presentation. McNamara L. 25 October 2023. Plan for revisiting the adolescent schedule for meningococcal vaccines. (Link)
- ACIP Presentation. Collins J. 25 October 2023. Summary of EtR and proposed recommendations for Pfizer’s MenABCWY vaccine. (Link)
If you have any questions about these updates or would like to discuss this content, please don’t hesitate to reach out to me. .
Sincerely,
Ferdaus Hassan
ferdaus.hassan@sanofi.com
|